Have you ever forgotten your sunscreen on a beach day and left the beach looking like a lobster? It is important to protect your skin from the sun’s harmful rays, but some of the products we use to do this may be harming marine creatures.
Ultra-violet filters (UVFs), or sunscreens, are substances that physically or chemically block ultra-violet (UV) light, which is the light that causes sun burns. Sunscreens that physically block UV light are referred to as mineral sunscreens, which are typically titanium dioxide or zinc oxide. These substances work by reflecting UV rays away from the skin. Chemical sunscreens on the other hand, block UV rays by absorbing the rays. These sunscreens include substances such as avobenzone, octinoxate, octocrylene, and oxybenzone.
Oxybenzone (also known as benzophenone-3 or BP-3) is a chemical sunscreen used in many popular sunblock products. The substance dissolves easily in water and fats and does not readily biodegrade, and because of this, can be persistent once introduced into the environment.
Oxybenzone has been found in tap water, swimming pools, wastewater, and natural water (seawater, rivers, lakes, and groundwater) globally. It is the most frequently detected sunscreen chemical in natural waters.
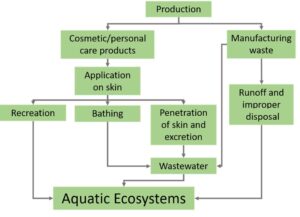
It is introduced into marine ecosystems when we apply it on our skin and then go swimming or play water sports. It is also washed off our skin and rinsed down the drain when we bathe. From the drain, it then travels through the collections system into a wastewater treatment plant. While many treatment plants are highly effective at treating wastewater, non-biodegradable substances such as oxybenzone prove challenging to remove. Therefore, they often pass through the treatment process and are discharged into waterways around the world.
Figure 1. Pathways that sunscreens enter the environment.Once introduced into the environment, oxybenzone then has the potential to negatively impact creatures who dwell within these marine habitats.
Coral
It is estimated that between 6,000 and 14,000 tons of sunscreen, containing 1–10% oxybenzone, is released into coral reef areas each year. There are numerous studies that show that oxybenzone can cause coral bleaching in hard corals, or the loss of a beneficial algae that live on and feed the coral. Coral bleaching leads to starvation and death of the coral. Oxybenzone does this by increasing the presence of viruses in the water that kill these vital coral-loving algae.
Oxybenzone is also a known endocrine disruptor, or a substance that alters the function of the body’s endocrine system. In high concentrations, exposure to this substance can cause hard coral to ossify, or harden into bone, as well as cause deformity, decreased mobility, and ultimately death.
Fish
Some fish are also impacted by oxybenzone. Studies in betta fish, rainbow trout, Japanese medaka, and zebrafish have shown evidence that it causes endocrine system disruption and reproductive harm. Oxybenzone has also been observed to cause behavioral changes, impaired development of reproductive organs in zebrafish, decreased egg production, and deformities in fish embryos.
Other Species
| Species | Impacts |
| Mussels | Bioaccumulation, or the buildup of a contaminant within an organism, toxicity and elevated stress. |
| Jellyfish | Bioaccumulation, behavioral changes, death |
| Green algae | Decreased growth rate, decreased biomass, cellular structure changes |
| Water fleas, clams, aquatic insects | Endocrine disruption, pregnancy inhibition, oxidative stress |
What can I do?
While chemical sunscreens can be detrimental to marine life, it is important to protect your skin from the sun. If you would like to protect both your skin and marine life, some options might be minimizing use of chemical sunscreens when possible and instead opting for protective clothing and hats. You may also wish to avoid spending time in the sun between 10 am and 4 pm, when the sun’s rays are most intense, or choose to stay in the shade.
When staying in the shade is not an option, you could strive to practice conscious consumerism and choose to purchase mineral sunscreen products that contain titanium dioxide and zinc oxide, rather than chemical sunscreens such oxybenzone and octocrylene. Be cautious of a “reef safe” label though. Some products make unfounded claims that their product is coral reef-friendly but contain various chemical sunscreen ingredients. You can practice due diligence and always check the ingredients before you buy!
While our understanding of how chemical sunscreens affect marine life is still in its early stages, a growing body of evidence suggests that it does indeed have a negative impact on many marine creatures. Next time you go to the beach, rather than turning into a lobster, you might want to take a moment to think about how your sunscreen could potentially impact lobsters and other creatures in the sea.
Blog post contributed by Lenzie Ward, P3 Specialist with HRSD.